Describe the Orbitals Used by the Carbon Atom in Bonding
The remaining sp orbitals form σ bonds with hydrogen atoms. CH 3 O b.

2 2 Hybrid Orbitals Organic Chemistry 1 An Open Textbook
Each carbon atom still has two half-filled 2p y and 2p z orbitals which are perpendicular both to each other and to the line formed by the sigma bonds.

. Science Chemistry Organic Chemistry 8th Edition Describe the orbitals used in bonding and the bond angles in the following compounds. Solution for Describe the orbitals used by each carbon atom in bonding and indicate the approximate bond angles1. Four CH bonds result from the overlap between the C atoms sp 2 orbitals with s orbitals on the hydrogen atoms.
One CC σ bond results from overlap of sp 2 hybrid orbitals on the carbon atom with one sp 2 hybrid orbital on the other carbon atom. The pi bond between carbon and double bonded oxygen atom is formed by unhybridized p-orbitals of carbon and oxygen. Transcribed image text.
B The π bond is formed by the side-by-side overlap of the two unhybridized. In the ethene molecule C 2 H 4 there are a five σ bonds. Two are formed by overlap between sp2 orbitals with 1s.
Therefore the bond angles are close to 120circ. Part E Describe the orbitals used by each carbon atom in bonding and indicate the approximate bond anges. How many atomic orbitals are used to form hybrid orbitals.
The number of carbon atoms linked is. Science Chemistry QA Library Describe the orbitals used in bonding and the bond angles in the following compounds. Reset Help sp sp.
The two unhybridized p orbitals per carbon are positioned such that they overlap side by side and hence form two π bonds. When acting as a ligand to a metal centre carbon monoxide is capable of forming a metal-carbon σ bond via donation of a lone pair from carbon. A C H 3.
Most Help 1100 HCN CCHCO. This creates four equivalent sp 3 hybridized orbitals. And if one triple bond is made by a carbon atom then the orbitals involved are sp.
Describe the orbitals used in bonding and the bond angles in the following compounds. 0 8p H H. CH 3 O b.
The bond between carbon and single bonded oxygen atom is formed by hybrid orbital of carbon atom and hybrid orbital of oxygen atom. No on the basis of the fact that if all single bonds are made by a carbon then it utilizes its sP three hybrid orbital. According to MO theory one sigma orbital is lower in energy than either of the two isolated atomic 1s orbitals this lower sigma orbital is referred to as a bonding molecular orbital.
While if there is one double bond made by a carbon with other items then the hybrid orbitals involved are sP two. 2 rows The carbon has three sigma bonds. It can also accept π-electron density from filled d-metal orbitals into the CO π antibonding orbital.
Which hybrid orbitals are used by carbon atoms in the following molecules. The two carbon atoms of acetylene are thus bound together by one σ bond. Not all labels will be used.
CarbonC is the 6th element in the periodic table and its symbol is C. It uses its leftover p orbital to form the second bond to oxygen. Drag the appropriate labels to their respective targets.
These two perpendicular pairs of p orbitals form two pi bonds between the carbons resulting in. The triple bond consists of one sigma bond and two pi bonds. The sp hybrid orbitals of the two carbon atoms overlap end to end to form a σ bond between the carbon atoms Figure 4.
Drag the appropriate them to their respective bins. Because carbon forms a double bond we know that it uses s p2 orbitals as it does in ethene to bond to the two hydrogens and the oxygen. A molecule in which s p 2 hybrid orbitals are used by the central atom in forming covalent bond is.
Overlap of each of the hybrid orbitals with a hydrogen orbital creates. Because carbon is s p2 hybridized the bond angles are approximately 120circ. View solution In diamond crystal each carbon atom is linked with carbon atoms.
Therefore the bond angles are 1095circ 4. Sp Draw the Lewis structure for H2CO3. This article gives an idea about the electron configuration of carbon and the orbital diagram period and groups valency and valence electrons of carbon bond formation compound formation application of.
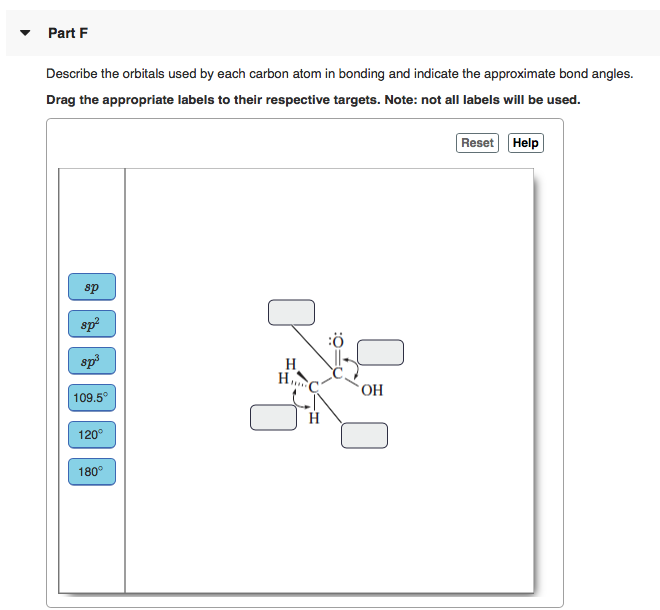
When two atomic 1s orbitals combine in the formation of H2 the result is two sigma σ orbitals. Draw the molecule by placing atoms on the canvas and connecting them with bonds. Part F Describe the orbitals used by each carbon atom in bonding and indicate the approximate bond angles.
SOLUTION TO 31 b. OH 1095 H 120 180. H 2 CO d.
A Lewis structure of H_2 C O_3 b the carbon uses s p2 hybridized orbitals. The four valence atomic orbitals from an isolated carbon atom all hybridize when the carbon bonds in a molecule like CH 4 with four regions of electron density. B the carbon uses s p3 hybridizied orbitals.
Molecular orbitals for H2.
Solved Part F Describe The Orbitals Used By Each Carbon Atom Chegg Com

0 Response to "Describe the Orbitals Used by the Carbon Atom in Bonding"
Post a Comment